CVN screenshot of plaintiffs' attorney Caleb Mason delivering his opening statement. Click here to see video from the trial.
Los Angeles, CA - A prominent plastic surgeon and a patient he treated with medical device manufacturer Cynosure’s “Cellulaze” fat removal laser system accused the company of being responsible for the patient’s burn injuries and falsely representing that the laser was approved by the FDA in a trial that began Thursday in California state court.
Dr. Stuart Linder, known for his appearances on several reality and news television shows, and Adriana Diaz sued Cynosure, a division of Hologic, in 2013, after a Cellulaze treatment on her leg allegedly left Diaz suffering from chronic pain, swelling and skin deformations.
Linder and Diaz claim Cynosure failed to test whether a temperature sensor on the laser accurately reflects how hot human tissue gets during its use, and that the company’s sales representatives made numerous misrepresentations when pitching the device, including claiming it had FDA approval for cellulite treatment.
Cynosure argues Linder should have known Diaz’s prior medical history made her a poor candidate for the treatment, and that their sales representatives told Linder the laser had FDA clearance, which is distinct from FDA approval.
The full trial, believed to be the first product liability claim involving the Cellulaze to go before a jury, is being webcast and recorded gavel-to-gavel by Courtroom View Network.
Linder’s and Diaz’s attorney, Caleb Mason of Brown White & Osborne LLP, told jurors that Cynosure originally designed the Cellulaze as a cutting laser but later decided to market it for cellulite treatment with some modifications.
Mason explained that Cynosure representatives reached out to Linder’s Beverly Hills practice with a pitch claiming the supposedly FDA approved device would generate substantial revenue.
“He would never buy a product and use a product that was not FDA approved,” Mason said.
After purchasing the device, Linder carried out his first Cellulaze treatment on Diaz, who worked as his office manager. Mason claimed the procedure, which occurred under the supervision of a Cynosure training nurse, took place consistent with Cynosure’s instructions.
The next morning Diaz awoke in severe pain with blood and fluid leaking from the area on her leg where Linder used the Cellulaze. Mason told jurors she suffers to this day from skin deformations called seromas, chronic swelling and pain, and weakness that makes exercise and other movements difficult.
Mason argued Cynosure never told Linder that patients who had previous liposuction, like Diaz, are poor candidates for Cellulaze treatment because of the lack of fatty tissue to absorb the laser’s energy.
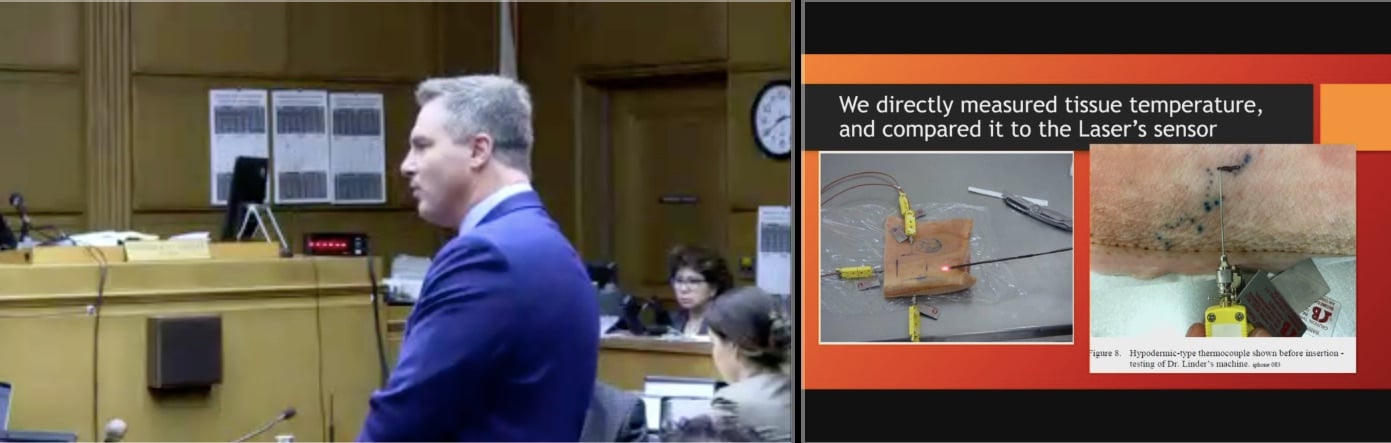
He also alleged that a sensor on the Cellulaze, which resembles a long, thin wand that is inserted into fat deposits, is defectively designed and registers much lower temperatures than are actually present in the tissue.
During a test of the device on pig tissue by an expert retained by the plaintiffs’ team, Mason said the internal temperature near the device registered at over 300 degrees and created the smell of frying bacon in the test laboratory.
Mason didn’t as for a specific amount of damages during his opening, but Linder and Diaz’s complaint asks for compensatory and punitive damages on 14 counts, including fraud, negligent misrepresentation, false advertising, conversion, product liability, breach of warranty and failure to warn.
Representing Cynosure, Karen Curtis of Gordon Rees Scully Mansukhani LLP told jurors during her opening that all surgical procedures carry some risk, and that side effects like seromas are commonly associated with laser fat treatments.
She also argued Diaz and Linder didn’t disclose her full medical history to the Cynosure reps consulted prior to her treatment.
“Ms. Diaz never told anyone at Cynosure that she had a prior liposuction and neither did Dr. Linder,” Curtis said.
In attempting to refute the allegations regarding FDA approval, Curtis explained the Cellulaze qualifies as a “Type 2” device under FDA regulations and does not require “approval” the way “Type 3” products, which include lifesaving devices, do.
Type 2 products go through a less rigorous “clearance” process, and Curtis told jurors she would present evidence clearly showing that Cynosure made accurate representations to Linder regarding the FDA’s evaluation of the Cellulaze.
Prior to the start of the trial Cynosure filed a motion seeking to bar all members of the news media from attending the public trial proceedings, however Judge Frederick Shaller rejected the request.
“[C]onducting a trial in private will impermissibly infringe on the right of the media to report newsworthy facts to the interested public and the factors in favor of allowing cameras, recording, and broadcasting outweigh any interest in the parties in an order that the proceedings be held in private,” Shaller wrote in his order denying Cynosure’s request.
Judge Shaller also rejected a request for a gag order to bar trial participants from discussing the case publicly.
“Prior restraints, such as the one contemplated by this request, are disfavored and presumptively invalid,” he wrote.
With both sides planning to extensively rely on expert witness testimony, the trial is expected to take up to five weeks to complete, and the full proceedings will be available live and on demand via CVN.
The case is captioned Adriana Diaz v. Cynosure Inc., case number BC559399, in Los Angeles County Superior Court.
E-mail David Siegel at dsiegel@cvn.com





