 CVN screenshot of defense attorney Dennis Ames delivering his opening statement
CVN screenshot of defense attorney Dennis Ames delivering his opening statement
Los Angeles - A California state court jury heard opening statements on Friday in the first lawsuit over alleged "off-label" use of medical device giant Medtronic Inc's "Infuse" spinal implant.
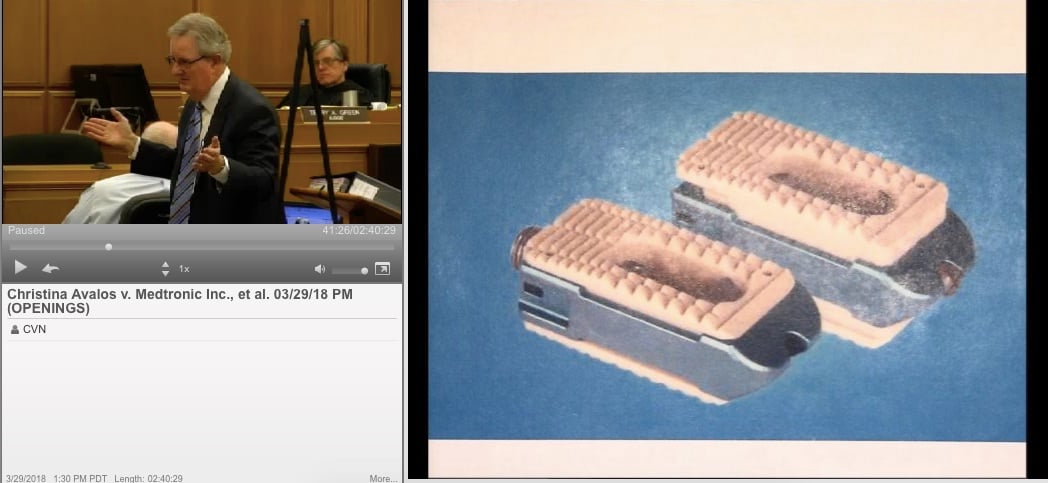
Plaintiff Christina Avalos accuses surgeon William Dobkin of using the Infuse device during lumbar surgery without her consent. The Infuse implant contains proteins meant to stimulate rapid bone growth after surgery, but Avalos claims the use of the Infuse in concert with another spinal implant called a “cage” resulted in excessive bone growth and inflammation that left her suffering from disabling pain.
While Medtronic has been dismissed and is no longer an active party in the case, the trial is believed to be the first time claims involving supposed off-label use of the Infuse device will go before a jury. Medtronic faced roughly 6000 actual and threatened lawsuits related to the Infuse but claims to have resolved most of them after setting aside $300 million in settlement funds.
Avalos argues in court papers that Dobkin committed medical battery when he used the Infuse device along with a cage manufactured by Globus Medical Inc., and that Medtronic’s investment in a medical software company he founded created a conflict of interest.
Dobkin has stated that he did obtain the proper consent from Avalos, and that he had no relationship with Medtronic or Globus that influenced his treatment decisions.
The Infuse device received FDA approval in 2002 for use in specialized back surgeries, but doctors soon began using to treat a wider variety of more common conditions. Off-label use is legal in the United States, because the FDA regulates medical devices and not the actual treatment that patients receive.
Medtronic allegedly purposefully marketed Infuse for off-label use even as increasing evidence of adverse outcomes piled up, and in 2006 the company paid $40 million to settle a Justice Department probe of a whistleblower’s claims that the company paid “kickbacks” to doctors to encourage the use of Infuse.
Medtronic denies the allegations, maintaining that it complied with the law while promoting Infuse and that it can’t control if doctors independently choose to use it in an unapproved way.
The trial will take place before Los Angeles Count Superior Court Judge Terry Green, and is expected to take approximately three weeks to complete. CVN subscribers will have gavel-to-gavel live and on demand access to the proceedings, along with numerous other medical malpractice and medical device product liability trials in CVN’s online trial video archive.
Avalos is represented by Ronald L.M. Goldman and Bijan Esfandiary of Baum Hedlund Aristei & Goldman PC.
Dobkin is represented by Dennis Ames and Michael Doubet of La Follette Johnson De Haas Fester & Ames.
The case is captioned Christina Avalos v. Medtronic Inc., et al., case number BC537742.
Email David Siegel at dsiegel@cvn.com





